Spotlights
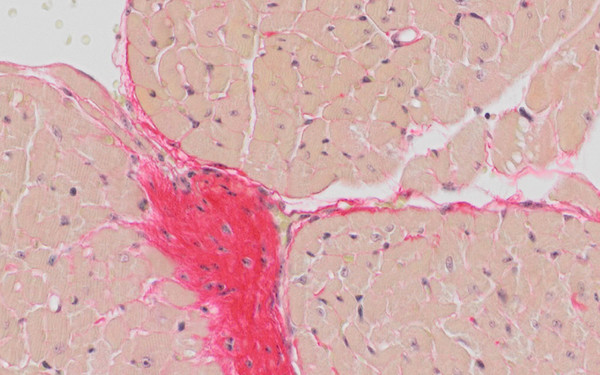
Stressed vessels in progeria
LMNA mutations cause progeria, a severe disease with features of accelerating aging in multiple tissues, including cardiovascular disease and atherosclerosis. We show that vascular endothelial cells in progeria cannot cope with blood flow-mediated shear stress and upregulate pro-fibrotic mechanosignaling pathways, causing fibrosis, vessel stiffening and heart problems.
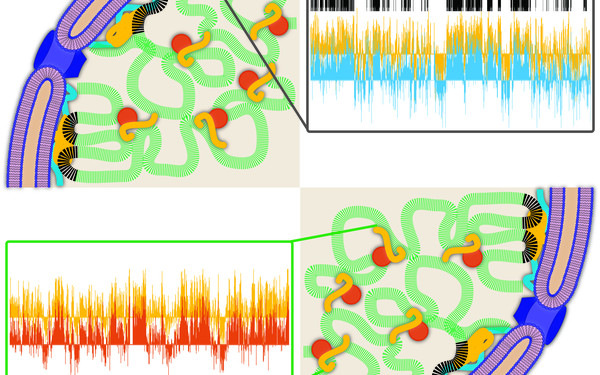
Open-up chromatin
While the attachment of heterochromatin to the peripheral lamina is well known, we found that, in addition, lamins bind to open, active chromatin regions inside the nucleus. This novel and dynamic lamin-chromatin interaction increases chromatin accessibility and regulates epigenetic pathways thereby contributing to differentiation-specific gene expression.
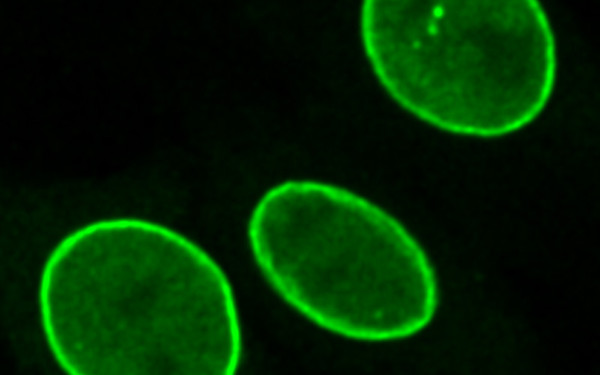
Lamins – a life outside the lamina
In addition to their peripheral localization, lamins are found in a dynamic pool in the nuclear interior. We identified the lamin-binding protein,LAP2alpha,as a cell cycle-dependent regulator of the dynamic lamin pool. Depletion of LAP2alpha leads to loss of nucleoplasmic lamins and impairment of adult stem cell regulation.
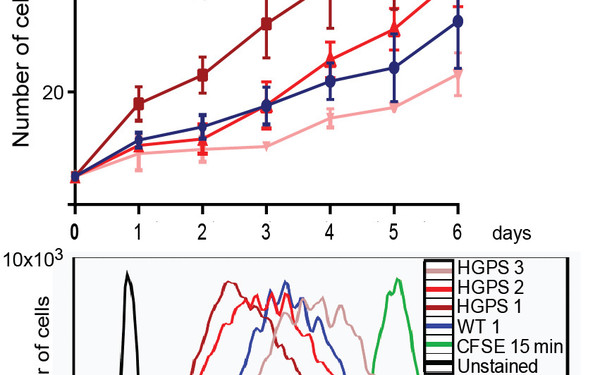
Rejuvinating aged cells
Impaired cell proliferation is a hallmark of premature aging disease and normal aging. We found that this is accompanied by loss of the nucleoplasmic lamin-LAP2alpha complexes. Re-expression of LAP2alpha in aged cells is sufficient to rescue proliferation defects, likely by inducing collagen expression. Our studies reveal potential new treatment strategies for age-linked diseases.