Spotlights
ERAD of a model substrate protein
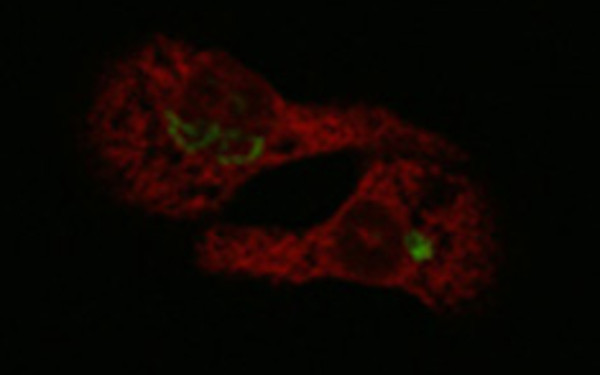
We have shown that a truncated form of ribophorin I, a model glycoprotein for ERAD, is degraded by the ubiquitin/proteasome system. The role of N-linked glycans in ERAD was pinpointed as temporary retention devices in the ER. Thus, interaction of N-glycosylated substrates with the calnexin cycle and perhaps other ER constituents appears to prolong their half lives.
The role of MTP and PDI in the assembly and secretion of atherogenic lipoprotein particles
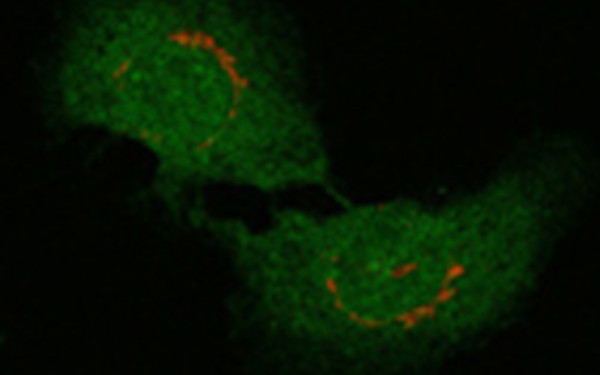
Microsomal triglyceride transfer protein (MTP) is a lipid transfer protein required for the assembly and secretion of very low density lipoproteins (VLDL). Active MTP is a heterodimer containing a 97 kDa catalytic subunit and a 58 kDa subunit (protein disulfide isomerase (PDI)). The MTP complex catalyzes the loading of apolipoprotein B (apoB) with lipids. In avians, the synthesis of VLDL is inducible by estrogen. We are studying the effect of estrogen treatment on MTP activity and on the regulation of VLDL secretion. The consequence of altered intracellular MTP activity on VLDL assembly and secretion is being analyzed. Another aspect is concerned with the mechanism of retention of the MTP complex in the ER.