Spotlights
Plectin, a global player in cytoskeleton organization
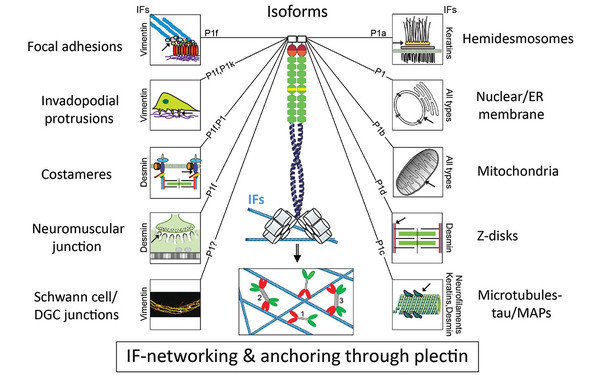
Plectin’s isoform diversity is based on alternative splicing of multiple first exons leading to the expression of protein variants with unique N-terminal domains. These domains specify distinct interaction partners, enabling differential isoform targeting. As all isoforms are endowed with a universal, high affinity IF-binding domain at their C termini, they recruit and anchor IF networks of any type to their target sites. The cell type and developmental stage dependent expression of isoforms in different combinations and proportions leads to isoform-dependent interlinking of different cellular structures and organelles, with consequences for cytoarchitecture, cell-cell and cell-matrix interactions, signaling, and migration potential of cells. In addition, plectin consolidates IF networks physically by filament crosslinking. (Wiche et al, Curr Opin Cell Biol 2015)
Myofibrillar myopathies
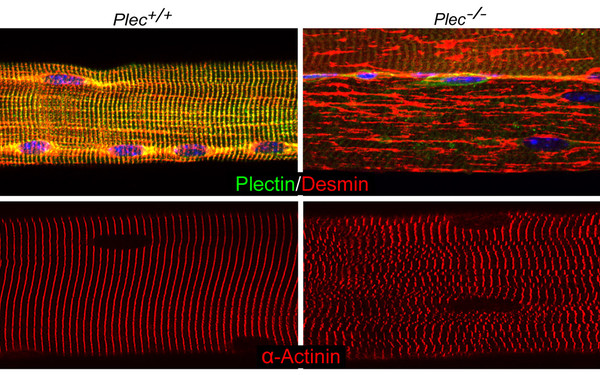
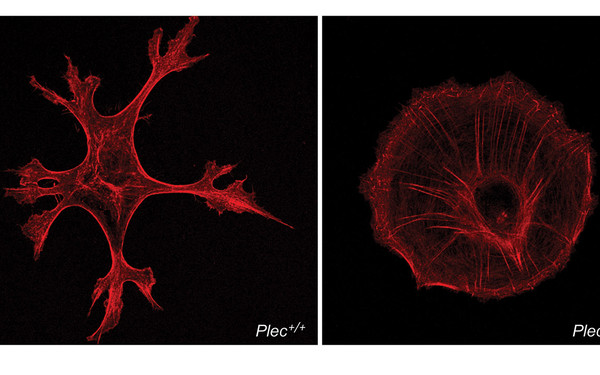
Plectin deficiency in skeletal muscle leads to myopathies manifesting with IF network collapse, protein aggregate formation, misalignment and sarcolemma-decoupling of myofibrils, dislocation and dysfunction of mitochondria, as well as structural and functional distortions of nuclei and neuromuscular synapses. These pleiotropic effects are due to the loss of one, or more, of the four major plectin isoforms that in mature myofibers are differentially targeted and recruiting desmin IFs to the sarcolemma, contractile apparatus, myonuclei, and mitochondria. Using differentiation competent myoblast cell cultures established from plectin-KO mouse muscle, we found a chemical chaperon (4-PBA) that alleviates protein aggregation and increases muscle strength in mice, setting the stage for clinical studies. (Winter et al, J Clin Invest 2014)
Skin - Epidermolysis bullosa simplex
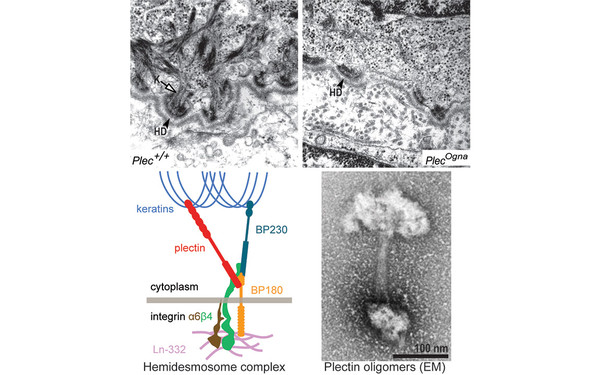
Skin blistering is the hallmark of most plectinopathies. We showed that plectin is a crucial component of the hemidesmosome (HD) junctional complex and a direct binding partner of integrins. The loss of isoform P1a leads to disruption of the connection between the intracellular keratin filament bundles (K) and the extracellular matrix, resulting in nonfunctional and less HDs. The analysis of transgenic mice mimicking the dominant human plectin EBS-Ogna mutation provided new insights into pathomechanisms and revealed a general HD-stabilizing mechanism based on lateral self-association of plectin’s ~200 nm long α-helical rod domains (see oligomerized plectin molecules). (Walko et al, PloS Genet 2011)
Astrocytes, learning & memory
Astrocytes play a prominent role in many brain activities. With their unique star-shaped morphology, astrocytes enwrap synapses and assume various anatomical links with neuronal processes, thereby affecting the formation and stability of synapses in their response to neuronal activity. There is evidence for an involvement of plectin in astroglia disorders, such as Alexander disease, and a significant upregulation of plectin has been observed in patients with temporal lobe epilepsy or a history of psychosis; plectin is also substantially increased in Alzheimer’s hippocampus. We have shown that P1c, the plectin isoform associating with microtubules, is implicated in axonal vesicle transport and long-term memory formation (Valencia et al, Neuropathol Appl Neurobiol. 2021). Thus, it will be a challenging task to establish mechanistic links between astrocyte-based neurologic diseases, cognitive dysfunctions, and plectin-related cytoskeleton deregulation.