

In the early 20th century, a German doctor observed unusual protein aggregates in the brain that would come to be known as Lewy bodies, a common feature of several neurodegenerative diseases. A major component of Lewy bodies is alpha synuclein, a poorly understood protein that has since become a therapeutic target. “Alpha synuclein is one of the most abundant proteins in the brain and aggregates of the protein are a hallmark of Parkinson’s,” says Thomas Schwarz, a postdoc in the Konrat lab and co-corresponding author of the study. “Although the pathological relevance of alpha synuclein is well established, both the mechanism by which it causes toxicity and its physiological function are still unclear”.
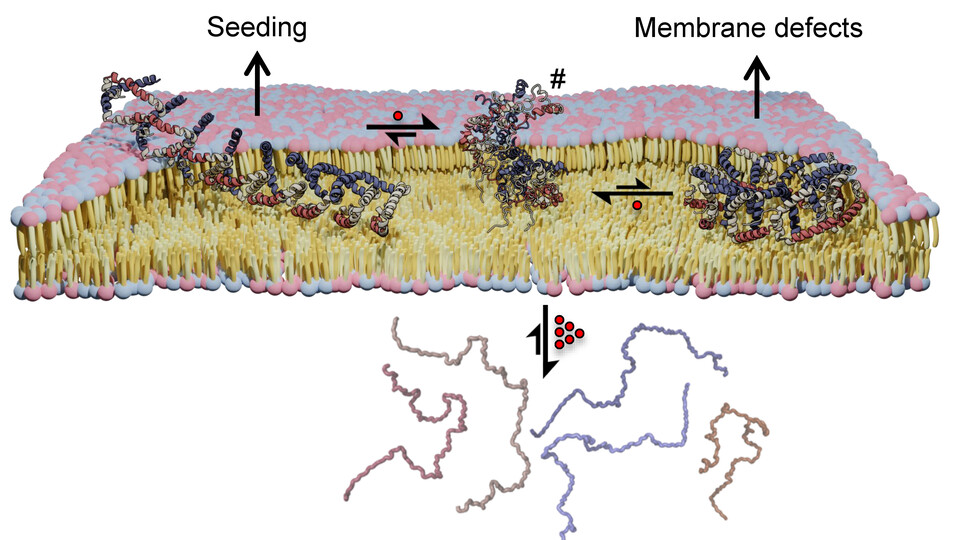
Alpha synuclein is what is known as an intrinsically disordered protein, or IDP. However, when bound to membranes, it adopts a stable, folded conformation that forms oligomeric assemblies involved in neuronal vesicle trafficking. When these protein assemblies are altered, grow much larger, and leave the membrane, alpha synuclein can form aggregates characteristic of Parkinson’s disease. Whether the membrane-bound aggregates are themselves toxic is controversial. Structural characterization of membrane-bound alpha synuclein could, however, help to explain the biological functions of alpha synuclein and provide a glimpse into its pathophysiology.
The team now used a combination of nuclear magnetic resonance (NMR) spectroscopy and cross-linking mass spectrometry (XL-MS) in collaboration with the Mass Spectrometry Facility at the Max Perutz Labs, to gain a deeper understanding of how membrane-bound alpha synuclein molecules assemble into higher order oligomers. They identified two distinct assemblies of alpha synuclein: a ring-like structure that exhibits features that may aid membrane insertion and an extended assembly that may facilitate its aggregation. “Our structural data also provide a possible explanation for known familial gene mutations that are associated with a higher risk of Parkinson’s disease,” explains Robert Konrat.
The scientists teamed up with the pharmaceutical company UCB to understand the mode of action of a potential Parkinson’s drug. A precursor compound to UCB0599 effectively clears membrane-bound alpha synuclein aggregates in mouse models. The scientists now demonstrate that UCB0599 disrupts membrane-bound alpha synuclein. “We observed that UBC0599 loosens the interactions between individual alpha synuclein molecules in the studied oligomers, which provides a plausible mode of action of the drug,” explains Thomas Schwarz. The team hopes that these findings will now aid in the further development of UCB0599 and its use. “Our findings will help to plan the decisive phase 3 trial, particularly with regard to dosage and development of efficient tests to probe biological efficacy,” says Robert Konrat.
Publication:
Thomas C. Schwarz, Andreas Beier, Karin Ledolter, Thomas Gossenreiter, Theresa Höfurthner, Markus Hartl, Terry S. Baker, Richard J. Taylor, and Robert Konrat: High-resolution structural information of membrane-bound α-synuclein provides insight into the MoA of the anti-Parkinson drug UCB0599. Proceedings of the National Academy of Sciences. 2023
https://doi.org/10.1073/pnas.2201910120