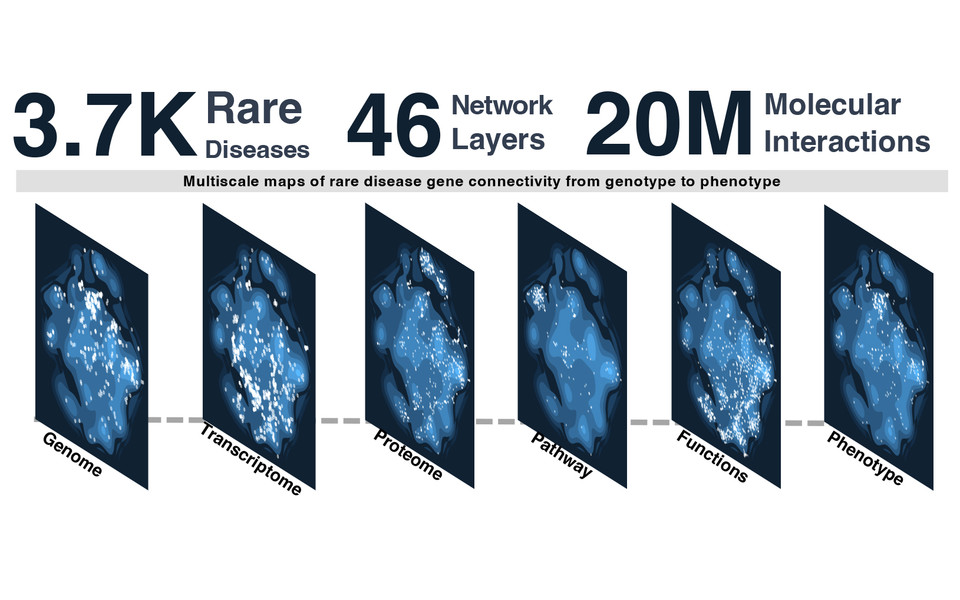
In contrast to common diseases, which are usually characterized by a complex interaction of multiple genetic and environmental factors, rare diseases can often be traced back to a single defective gene. Targeted decoding and analysis of a gene defect and its phenotypic consequences provides important information for understanding the underlying mechanisms and helps in choosing targeted treatment strategies. However, the individual search for the cause of disease is usually lengthy and costly. Jörg Menche’s research group has been applying molecular network analysis to improve the understanding of genetic interactions in rare diseases.
In their current study, first author Pisanu Buphamalai built a multilayer network, mapping more than 20 million gene relationships with information ranging from protein interactions to phenotypic similarities. To achieve this, the scientist integrated a comprehensive dataset of more than 3,700 rare diseases with a known genetic basis. "The multiplex network integrates different network layers that map different levels of the biological organization of our body, from the genome to the transcriptome, proteome and phenotype. By mapping protein interactions and mechanisms, we can also better characterize those proteins whose roles in disease were previously unknown and thus track down gene defects more efficiently”, explains Jörg Menche. “Our network approach increases the probability of finding the crucial gene aberration threefold compared to when these networks are considered separately”, adds Pisanu Buphamalai.
On the one hand, the multiplex network's modular design makes it possible to quantify the impact of a particular rare disease on a specific level of biological organization. This means determining whether certain cells, tissues, or organs are particularly affected by a genetic defect. On the other hand, the importance of certain molecular processes for a disease can also be measured. "It is precisely because of its complexity, the linking of molecular sequences and processes, that our multiplex network is significantly more powerful and successful than looking at each network individually. It also makes it easier to make predictions about the possible consequences of the genetic defect”, says Jörg Menche. The network was successfully tested for functionality in collaboration with Vanja Nagy at the Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases using data from patients with neurological diseases whose underlying genetic defect was already known. "Our study shows how a huge dataset can be used in the context of network medicine to address several practical and conceptual challenges in rare disease research to improve diagnosis and treatment for the benefit of patients", concludes Menche.
Pisanu Buphamalai, Tomislav Kokotovic, Vanja Nagy & Jörg Menche: Network analysis reveals rare disease signatures across multiple levels of biological organization. Nature Communications 2021.
https://doi.org/10.1038/s41467-021-26674-1