For many years, scientists have observed paradoxical behavior of stem cells. In their differentiated offspring, signaling molecules called interferons are activated upon viral infection and initiate a signaling cascade that results in the expression of antiviral genes, or interferon stimulated genes (ISGs). Stem cells, on the other hand, do not respond to interferon stimulation but are still remarkably resistant to viral infection. Accumulating evidence has indicated that the innate immune system, including ISGs, is constitutively turned on in stem cells. Precisely how the expression of ISGs is controlled in the absence of interferon signaling, however, has remained enigmatic.
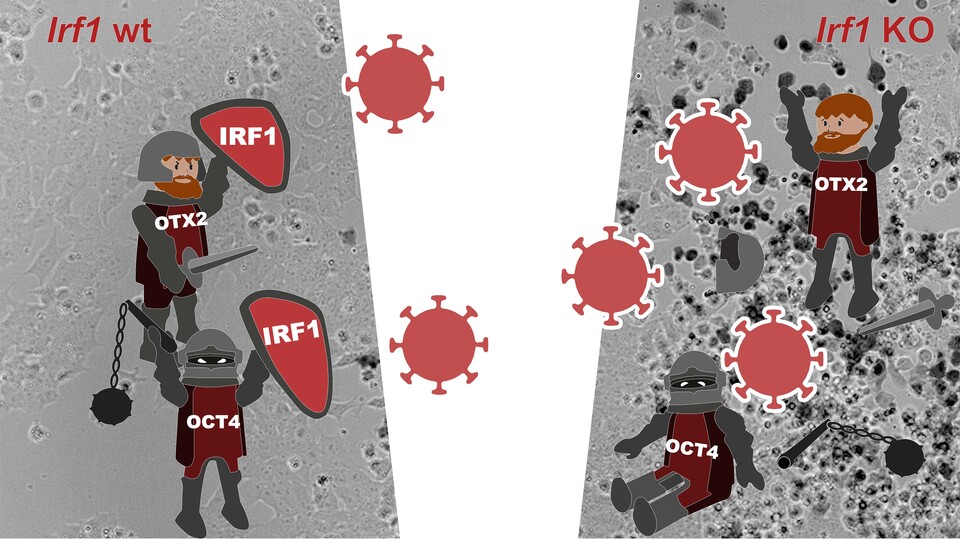
Merrit Romeike, a PhD student in the Bücker lab, first set out to identify factors that are involved in the exit from naïve pluripotency, a key developmental step of stem cells. To this end she performed a CRISPR knockout screen to identify factors that influence the exit from pluripotency. One of the top hits from the screen was a gene called Irf1, an ISG which encodes a transcription factor. Utilizing a simplified in vitro differentiation system with mouse embryonic stem cells, the scientist discovered that while it does not directly control the cell state transition, it is nevertheless connected to the genetic network at the heart of the pluripotent cell state. “We could show that Irf1 is directly controlled by two pluripotency genes called Oct4 and Otx2 through a stem cell-specific enhancer”, explains Merrit Romeike.
Oct4 and Otx2 are both important transcription factors that safeguard the pluripotent cell state. Irf1 in turn controls a subset of ISGs in stem cells that confer antiviral protection. “The same genes that help cells to keep their identity also upregulate the genes which are required for the viral defense”, says Merrit Romeike. In vivo data further show that this intrinsic upregulation is transient and confined to a short timeframe. “Before implantation of the embryo into the uterus it is surrounded by the zona pellucida, an extra layer that protects the embryo for example from viral infection. But during implantation, the embryo hatches from that layer and attaches to the uterine wall. We found that exactly during this period of hatching and implanting, the intrinsic network upregulates Irf1“, says Merrit Romeike. “We suggest that, in vivo, the prophylactic priming of the antiviral defense system might protect the embryo at this vulnerable moment in development”. However, further research is necessary to confirm this observation in living organisms.
Publication:
Merrit Romeike, Stephanie Spach, Marie Huber, Songjie Feng, Gintautas Vainorius, Ulrich Elling, Gjis A Versteeg, Christa Buecker: Transient upregulation of IRF1 during exit from naive pluripotency confers viral protection. EMBO Reports 2022