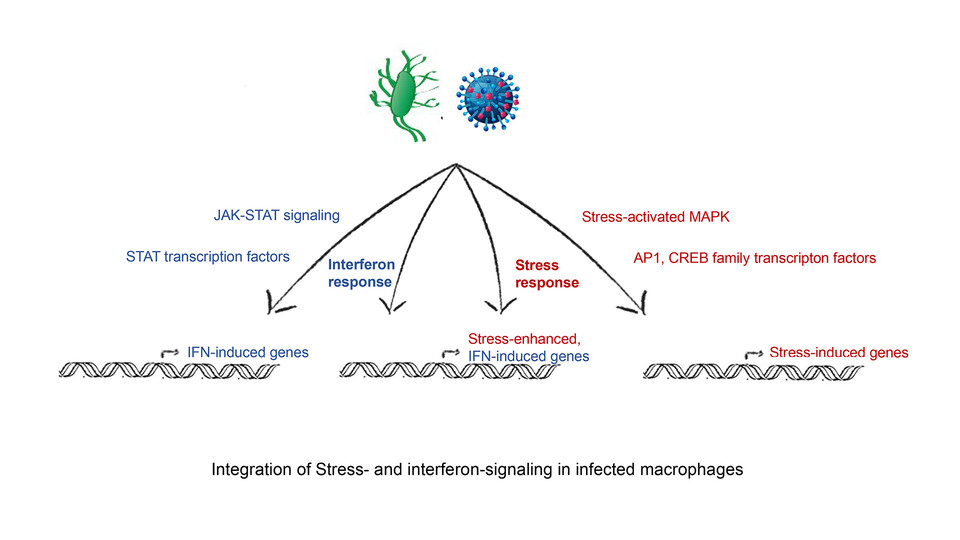
Cells adapt to their environment by mounting appropriate responses to external and internal cues. During infection, cells upregulate genes that help them to combat the pathogen. This defense system is activated by immunological mediators, such as interferons, through an intricate system of receptors, ligands, and signaling molecules. But interferon signaling is not the only pathway active in the course of an infection. “Different signaling pathways have been extensively studied independently of each other”, says group leader Thomas Decker. “In living cells, however, many pathways are turned on simultaneously during infection. To understand the response of a cell to infection, you have to look at the cross-talk between them.” The Decker lab now provides the first glimpse into how the stress-induced p38/MAPK pathway modulates interferon signaling.
Working with scientists from the University of Veterinary Medicine, Vienna and the Medical University of Vienna, the team performed a genome-wide analysis which revealed that a subgroup of interferon-stimulated genes was up-regulated when the stress pathway was simultaneously activated. “Those interferon-stimulated genes that are enhanced by stress have binding sites for transcription factors activated by the stress pathway”, explains Thomas Decker. The scientists were surprised by the extent of the effect: “Of the hundreds of genes induced by interferons, a large fraction was enhanced when there was a concomitant stress response”, says Thomas Decker.
Using infection models, the researchers tested the effect of stress signaling on the immune response. In macrophages infected with the bacterium Listeria monocytogenes, stress signaling increased cell death by driving the overexpression of genes that increase the production of nitric oxide. The team discovered that blocking the p38/MAPK pathway with inhibitors improved the viability of macrophages. Innate immunity has long been known to be a double-edged sword, and an excessive immune response can lead to damage for the host. The scientists’ discovery of interferon-stimulated genes that are hyper-activated by cellular stress could, therefore, provide potential therapeutic targets in patients with hyperinflammatory syndromes.
Laura Boccuni, Elke Podgorschek, Moritz Schmiedeberg, Ekaterini Platanitis, Peter Traxler, Philipp Fischer, Alessia Schirripa, Philipp Novoszel, Angel R. Nebreda, J. Simon C. Arthur, Nikolaus Fortelny, Matthias Farlik, Veronika Sexl, Christoph Bock, Maria Sibilia, Pavel Kovarik, Mathias Müller, and Thomas Decker: Stress signaling boosts interferon-induced gene transcription in macrophages. Science Signaling 2022
DOI: 10.1126/scisignal.abq5389