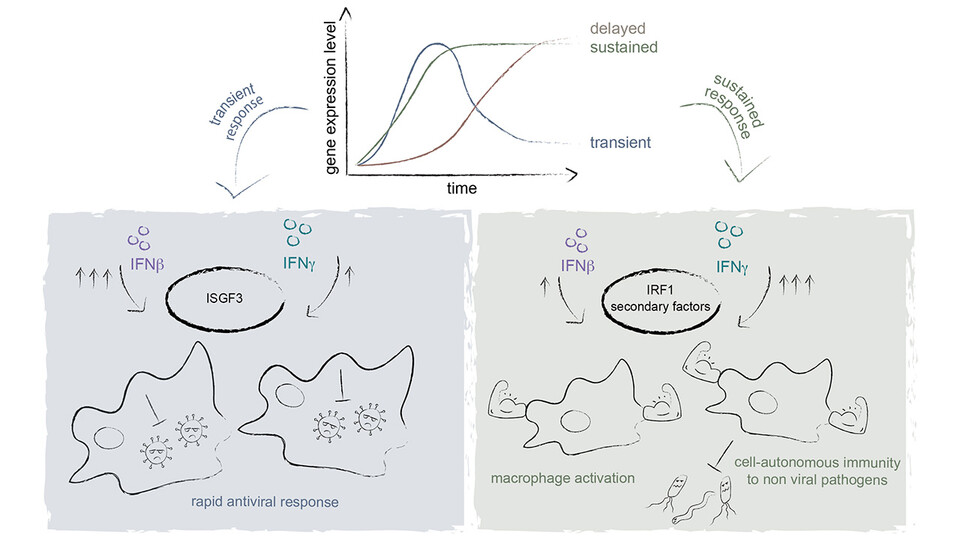

Interferons (IFNs) are cytokines with immune-modulating properties that contribute to the defense against viruses and other pathogens as part of the innate immune response. Type I interferons (IFN-I), such as IFNβ, are known for their antiviral effects, while type II interferons (IFN-II), including IFNγ, primarily activate macrophages. Their different biological outcomes are the result of subtle differences in the same signaling pathway. Both IFN-I and IFN-II are known to activate the JAK-STAT signaling pathway, crucial for regulating gene transcription via protein tyrosine kinases (JAKs) and Signal Transducers and Activators of Transcription proteins (STATs). IFN-I prompts the heterodimerization of STAT1 and STAT2, leading subsequently to the activation of interferon-stimulated factor 3 (ISGF3), enhancing the transcription of interferon-stimulated genes (ISGs). On the other hand, IFN-II activates a STAT1 homodimer, stimulating ISG transcription from associated gamma-interferon-activation side (GAS) through the gamma-interferon activated factor (GAF).
Until now, how and where these two pathways diverge was not well understood. The Decker lab found that the answer lies with the ISGF3 complex and the transcription factor IRF1. IRF1 can cooperate with GAF and ISGF3 and therefore plays a crucial role in pathways activated by IFN-β and IFN-γ. Perutz PI Thomas Decker elucidates: "Both interferon types trigger the expression of the IRF1 gene during the initial response. However, with IFNγ, IRF1 transcripts persist for an extended period. IRF1 therefore sustains the transcription of IFNγ-induced genes." Following the activation of IFNβ, however, IRF1 amounts subside rapidly, therefore shutting down IRF1-mediated transcription.
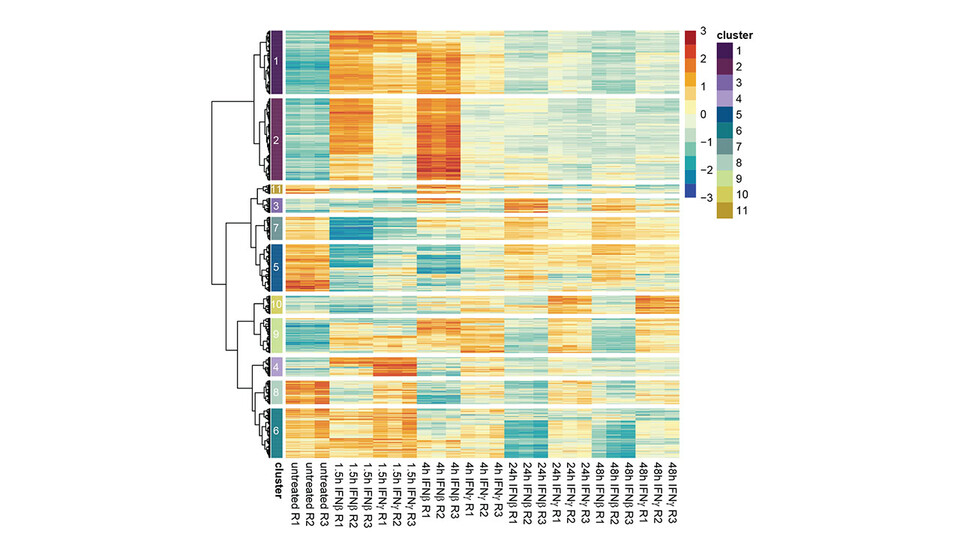
Thomas emphasizes the significance of their work: "The temporal evolution of the transcriptional profile reveals how the different interferons drive distinct transcriptional signatures." Initially, there is substantial overlap in the early stages of gene expression for both IFNs. However, as time progresses, the divergence increases, resulting in either the sustained expression of existing genes with IFN-II or the induction of new genes with IFN-I. The findings indicate that it is not just the initial pathway that influences the immunological response, but also time-dependent changes in the transcriptome.
To investigate the precise transcripts over certain time points, the Decker lab employed a technique called PRO-seq (Precision Run-On Sequencing). “Rather than capturing the entirety of RNA in a cell, transcribing it to cDNA and subsequently sequencing it, we utilize a technology that enables us to selectively observe only RNAs that are currently being transcribed, known as nascent RNA. This provides us with a snapshot of transcription activity, depicting what is actively occurring in the cell nucleus, as opposed to the total RNA content”, Thomas elaborates. Since the 1980s, interferons have been implicated in cancer and other diseases, but medicine has yet to fully realize their potential. The study of interferons and their signaling pathways will undoubtedly inform our understanding of disease pathogenesis and efforts to counter it in the clinic.
DOI: 10.1038/s44318-024-00092-7
About the Decker lab