
In contrast to most phases of a cell’s life, meiosis requires programmed double-strand DNA breaks. These double-strand breaks set the stage for crossing over – the exchange of maternal and paternal DNA that fuels genetic variation in offspring. Because of the potential for DNA damage and genome instability, the process of crossing over is tightly regulated: only a small fraction of breaks become crossovers, while the rest are simply repaired without the exchange of genetic material. The BTR protein complex, made up of the Bloom helicase, topoisomerases, and RMI scaffolding proteins, has been described to decatenate these junctions, thereby inhibiting crossover formation.
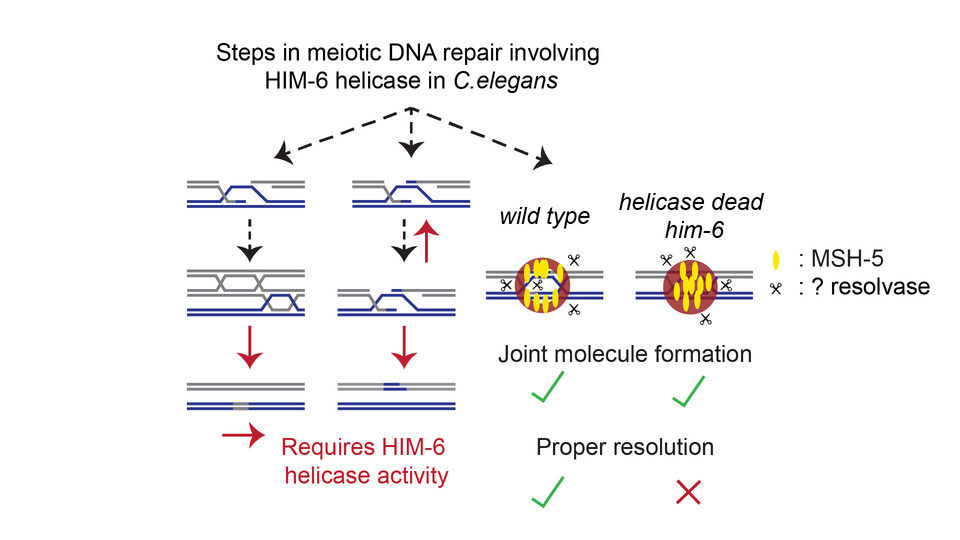
New findings, however, from the Jantsch-Plunger lab now show that the BTR complex is equally vital for ensuring that crossovers form correctly in Caenorhabditis elegans. This dual role was revealed when the team discovered that a catalytically inactive him-6 mutant strain was unexpectedly healthy. “The big surprise was that the catalytically inactive strain was extremely healthy – whereas the null mutant produced many unviable eggs, this strain looked almost like wild type”, explains first author and postdoc Sowmya Sivakumar Geetha. This suggested that HIM-6 must fulfill an additional, non-catalytic role in meiosis.
The researchers found, surprisingly, that even when HIM-6 cannot unwind DNA, it supports crossover formation at near wild-type levels. In contrast, him-6 null mutants exhibit markedly reduced numbers of crossovers. Although the unwinding activity of HIM-6 is apparently not necessary to support crossover formation, the scientists observed that its catalytic activity contributed to shaping the geometry of joint DNA structures called double Holliday junctions, ensuring that resolvases can properly cut the junctions to yield crossovers. The researchers propose that HIM-6 not only helps to provide a continuous flux of substrates for crossover formation but also biases the resolution of double Holliday junctions into crossovers.
Group leader Verena Jantsch-Plunger comments: “These findings suggest that current models based on reconstitution experiments may be over-simplified. Since Bloom helicase mutations cause Bloom Syndrome, a human disease characterized by developmental defects and a predisposition to cancer, deciphering its role and mechanism of action is crucial.” The study was a collaboration with Simone Köhler at the European Molecular Biology Laboratory (EMBL) Heidelberg.
DOI: 10.1093/nar/gkaf1030